Covid: Oxford jab offers less S Africa variant protection
- Published
Prof Sarah Gilbert said the new version of the vaccine "will be available for the autumn"
Oxford-AstraZeneca's vaccine offers "minimal protection" against mild disease from the South Africa variant, scientists say early trials suggest.
A new study, not yet peer reviewed, involved about 2,000 people who were on average 31 years old.
But Prof Sarah Gilbert, Oxford lead vaccine developer, said vaccines should still protect against severe disease.
She said developers were likely to have a modified Oxford jab by the autumn to combat the South Africa variant.
South Africa has said it is putting its rollout of the Oxford vaccine on temporary hold, while scientists advise on the best way to proceed following the results of the study.
The variant, also known as 501.V2 or B.1.351, is already the dominant virus variant in large parts of South Africa.
Earlier, vaccine minister Nadhim Zahawi told the BBC's Andrew Marr that a booster in the autumn and annual vaccines could be required to combat variants.
More than 100 cases of the South Africa variant have been found in the UK.
A study outlining early trials, first reported by the Financial Times, external, suggests the vaccine offered "minimal protection" against mild and moderate disease caused by the variant, the University of Oxford said.
Prof Gilbert told the Andrew Marr Show current vaccines "have a reduction in efficacy against some of the variant viruses".
"What that is looking like is that we may not be reducing the total number of cases but there's still protection in that case against deaths, hospitalisations and severe disease."
She added: "That's really important for healthcare systems, even if we are having mild and asymptomatic infections, to prevent people going into hospital with Covid would have a major effect."
Meanwhile, Mr Zahawi said the UK government had ruled out issuing so-called "vaccine passports" to enable people who have had the jab against coronavirus to travel abroad.


This isn't a complete surprise.
The mutation involved - known as E484K - seems to help the virus dodge immunity built up to previous variants and current vaccines.
Real world trials of two other vaccines (Janssen and Novavax) showed a dip in performance in South Africa too.
This is the same mutation that has also appeared in some cases of the "Kent variant" in the UK.
We are still waiting for scientists to publish their report in order to find out what "limited" means in hard numbers.
If people are still getting sick, even if it's mild, then they can spread the virus and that will make it harder to get on top of cases.
If the Oxford vaccine can prevent severe disease, and AstraZeneca thinks it will, then it would still be a life-saving vaccine.
But it is clear coronavirus is a moving target and that we may need to change vaccines in the future to keep up.

In a separate statement, AstraZeneca said the jab offered "limited" protection against mild and moderate disease caused by the variant.
A spokesman for the pharmaceutical company said it had not yet been able to properly establish whether the jab would prevent severe disease and hospitalisation caused by the South Africa variant because those involved in the study had predominantly been young, healthy adults.
But the company expressed confidence the vaccine would offer protection against serious cases, because it created neutralising antibodies similar to those of other coronavirus vaccines.
The study, which assessed a two-dose regimen, is due to be published on Monday.
Andrew Pollard, professor of paediatric infection and immunity and chief investigator on the Oxford vaccine trial, said: "This study confirms that the pandemic coronavirus will find ways to continue to spread in vaccinated populations, as expected, but, taken with the promising results from other studies in South Africa using a similar viral vector, vaccines may continue to ease the toll on health care systems by preventing severe disease."
In a news conference on Sunday, Prof Shabir Madhi, who has led trials for the Oxford-AstraZeneca vaccine in South Africa, said the study had been "largely disappointing" in terms of the jab's efficacy against the South Africa variant.
"Unfortunately, the AstraZeneca vaccine does not work against mild and moderate illness," he said, adding that the data did not say whether or not the vaccine might still protect at least against severe infections.
Earlier, Prof Madhi said a vaccine developed by Janssen showed it stopped moderate to severe disease, rather than all disease, and this kept people out of hospital.
"These findings recalibrate thinking about how to approach the pandemic virus and shift the focus from the goal of herd immunity against transmission to the protection of all at-risk individuals in population against severe disease," Prof Madhi added.

LOOK-UP TOOL: How many cases in your area?
OXFORD JAB: What is the Oxford-AstraZeneca vaccine?
QUARANTINE: Will I need to self-isolate in a hotel?

On Saturday, AstraZeneca said its vaccine provided good protection against the variant first discovered in Kent, which is now dominant in the UK.
Early results suggest the Pfizer-BioNTech vaccine protects against the new variants.
Data on two new coronavirus vaccines that could be approved soon - one from Novavax and another from Janssen - appear to offer some protection.
And early results from Moderna suggest its vaccine is still effective against the South Africa variant.
Experts say vaccines could be redesigned and tweaked to be a better match for new variants in a matter of weeks or months if necessary.
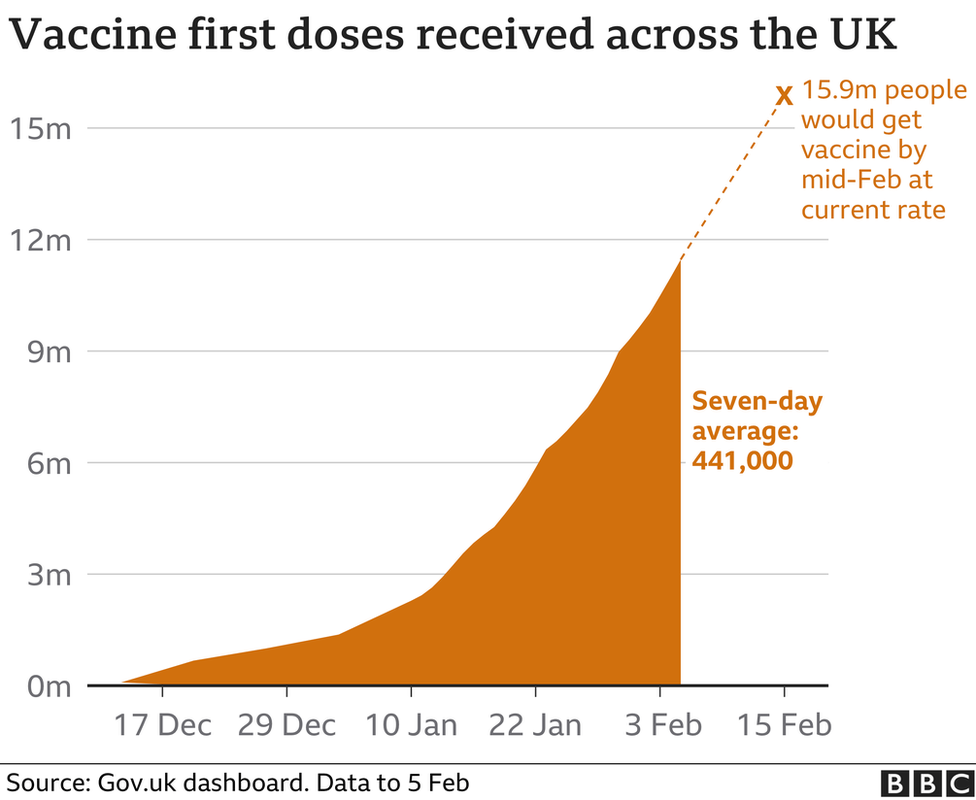

Meanwhile, workplace Covid testing is being offered to more companies in England and GPs will be paid an additional £10 by the NHS for every housebound patient they vaccinate
As of Friday more than 11.5 million people had received a first dose
Latest figures, external showed another 828 people in the UK have died within 28 days of a positive Covid test, and there were a further 18,262 cases.

THE THURSDAY MURDER CLUB: Escape with Richard Osman's bestselling crime novel...
RADIO 1'S CHILL MIX: Soothing musical vibes to soundtrack your weekend in lockdown

Have you been affected by issues relating to the vaccine rollout? Share your experiences by emailing haveyoursay@bbc.co.uk, external.
Please include a contact number if you are willing to speak to a BBC journalist. You can also get in touch in the following ways:
WhatsApp: +44 7756 165803
Tweet: @BBC_HaveYourSay, external
Please read our terms & conditions and privacy policy
If you are reading this page and can't see the form you will need to visit the mobile version of the BBC website to submit your question or comment or you can email us at HaveYourSay@bbc.co.uk, external. Please include your name, age and location with any submission.
- Published6 February 2021
- Published2 February 2021
- Published5 February 2021
- Published8 January 2021
- Published25 January 2021
- Published5 February 2021