Covid-19: Vaccine booster study begins in Cambridge
- Published

All trial participants will be aged 30 or older and will have already have had two doses of Covid-19 vaccine
Clinical trials have begun in Cambridge to see which Covid-19 vaccine works best as a third "booster" jab.
Researchers at the Addenbrooke's Hospital site are recruiting about 180 participants for a national trial, which will test seven vaccines.
The Cov-Boost study, external will give people a third dose of a vaccine to see whether it offers better protection against the virus than the standard two injections.
Prof Krishna Chatterjee called the study an "exciting opportunity".
The government-funded trial, led by the University of Southampton, is taking place at 18 sites across the UK and is said to be the first study in the world to provide vital data on the impact of a third dose on patients' immune responses.
It will look at seven Covid-19 vaccines - Oxford-AstraZeneca, Pfizer-BioNTech, Moderna, Novavax, Valneva, Janssen and Curevac - as potential boosters, given at least 10 to 12 weeks after a second dose.

'Helping millions of people'
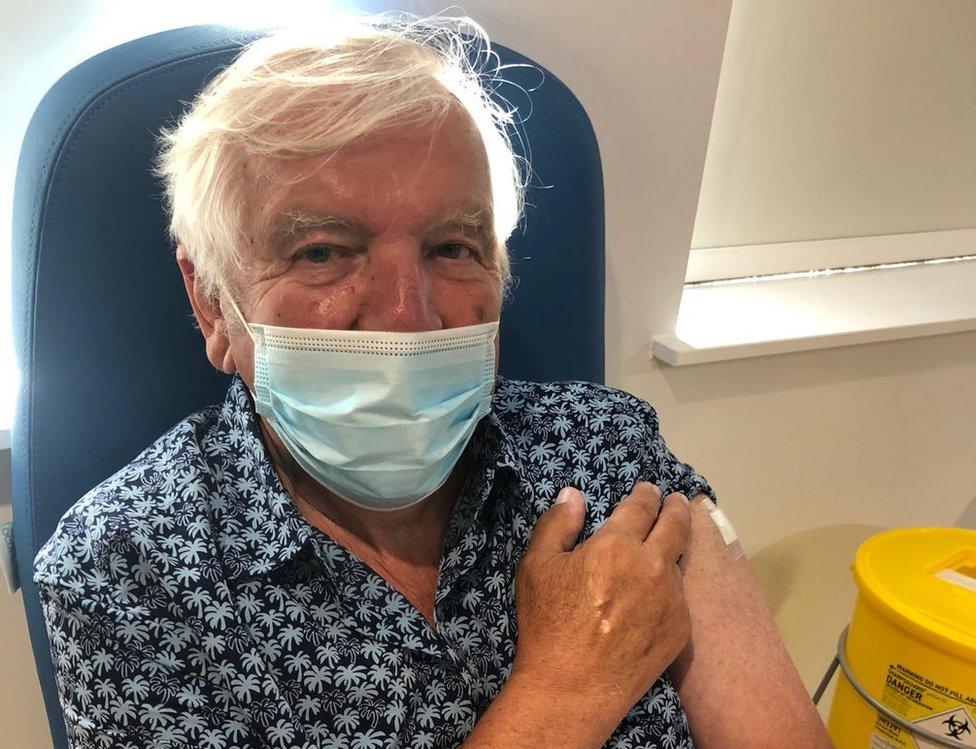
John Hodgson said the trial will help everyone in the end
John Hodgson said he hoped the trial could help "thousands, even millions, of people".
Mr Hodgson, 80, from Sawston near Cambridge, said he had been one of the "lucky ones" when he got his first two jabs in December and January.
"I feel privileged," he said. "We will all benefit in the end.
"If we can learn from this... I would have done my bit."
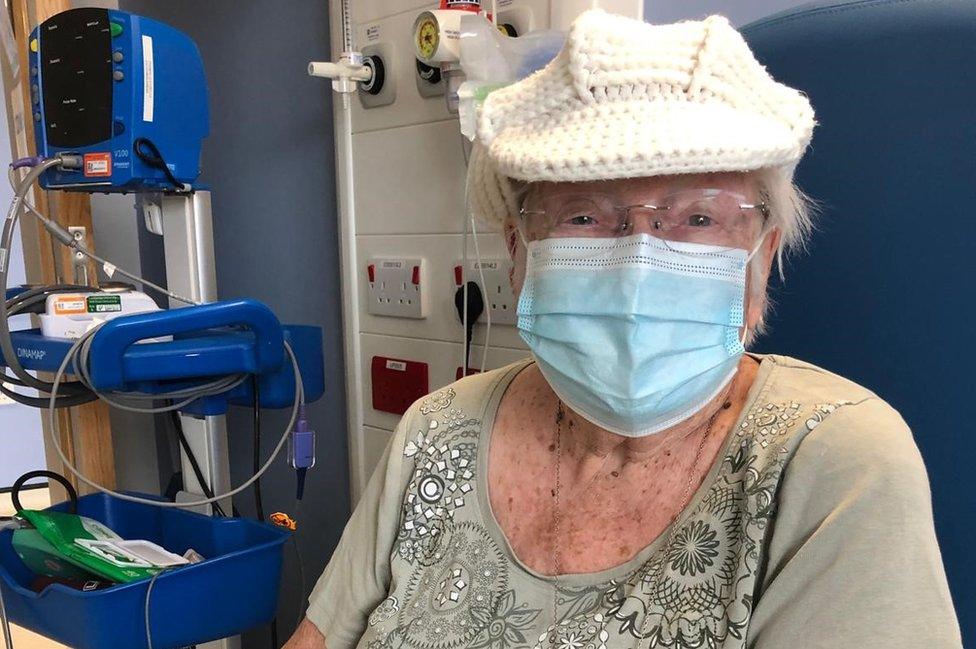
Audrey Philip said the pandemic has been worse than the second world war as people have been stuck in their homes for so long
Audrey Philip, from Dry Drayton, said at 88 years old "there is very little I can do, so I thought I should be putting something back to help research".
"I think it [the pandemic] is worse than the war as people are so confined," she said.
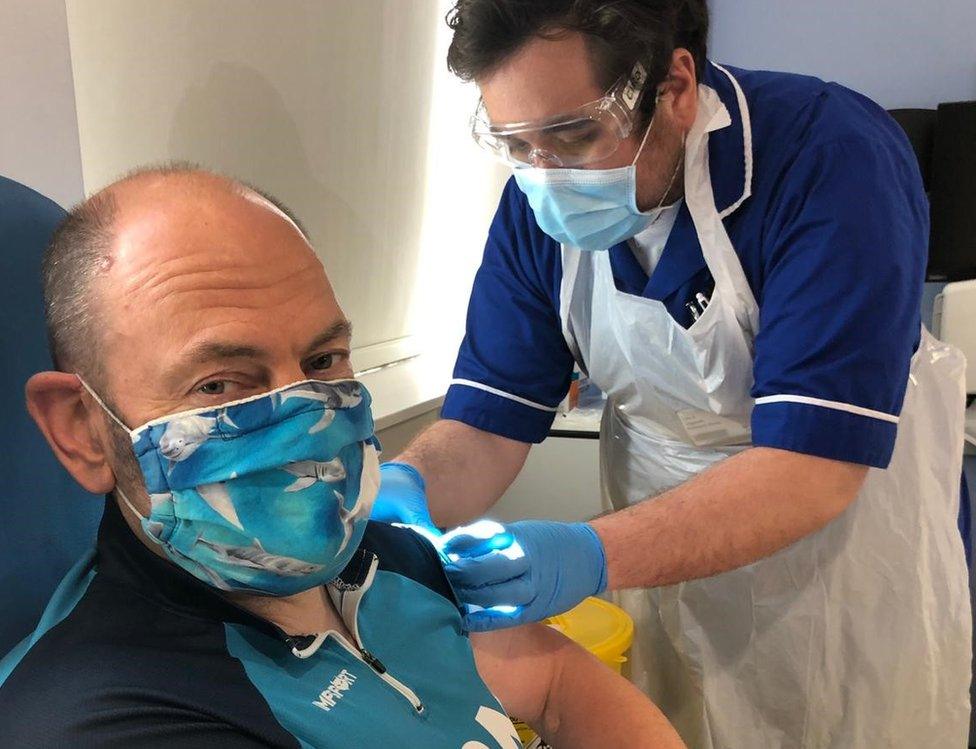
Andy Nightingale has worked as a volunteer for the ambulance service during the pandemic
Andy Nightingale was one of the first people in the country to get vaccinated because of his work with the volunteer ambulance service.
The 51-year-old said the trial was "very important".
"I just want to help where I can," he added. "I'm just hoping everyone else can benefit as a result."

Researchers at the National Institute for Health Research (NIHR) Cambridge Clinical Research Facility has been recruiting local volunteers, all of whom must be aged 30 or over and have already had two doses of a vaccine.

Prof Krishna Chatterjee said it was an "exciting opportunity to now work on a study to determine the effects of a third 'booster' dose"
They will each get one booster, either a placebo or a vaccine, which could be a different brand to the one they were originally vaccinated with.
Afterwards, they will be monitored for any reaction and their immune response will also be measured over the next year, although initial results from the data are expected in September.
This will help inform decisions by the Joint Committee on Vaccination and Immunisation (JCVI) on any potential booster programme from the autumn.

The NIHR Cambridge Clinical Research Facility is on the Cambridge Biomedical Research Campus
Prof Chatterjee, the director of the facility in Cambridge, said: "We have conducted trials of several Covid-19 vaccine studies over the last year.
"It's an exciting opportunity to now work on a study to determine the effects of a third "booster" dose of vaccines and I want to thank the trial participants and our staff who are helping with this important research."

Find BBC News: East of England on Facebook, external, Instagram, external and Twitter, external. If you have a story suggestion email eastofenglandnews@bbc.co.uk, external