Hand surgeon's career 'saved by gene-silencing drug'
- Published
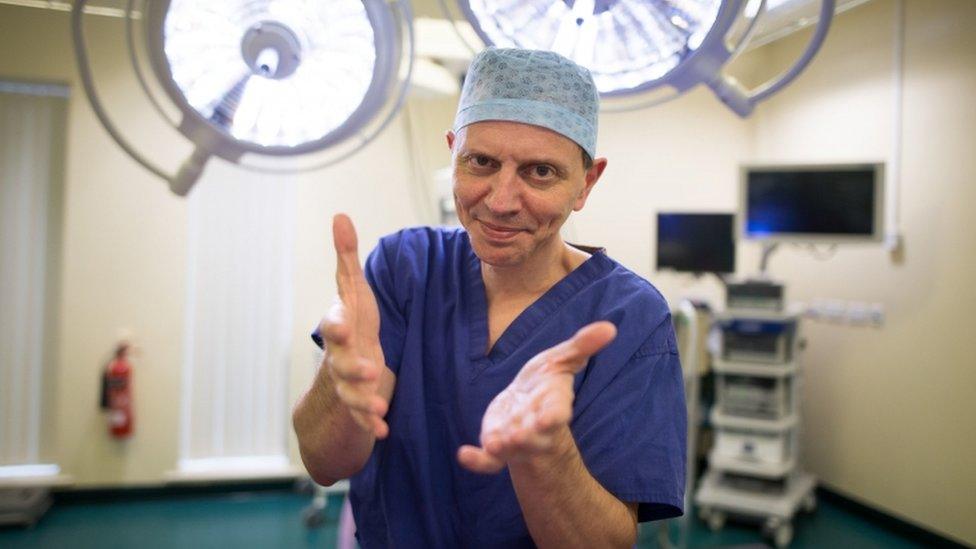
Hand surgeon Carlos Heras-Palou said his career "would have been finished" in six months without the treatment
A hand surgeon who faced losing the ability to perform operations said a new drug has saved his career.
Carlos Heras-Palou, an orthopaedic surgeon at Royal Derby Hospital, was diagnosed with transthyretin-mediated amyloidosis (hATTR amyloidosis).
The hereditary condition affects limb movement and other functions, and can be fatal in some cases.
The 53-year-old took part in a trial for patisiran, which blocks the activity of a gene in the liver.
Patisiran has now been licensed by European and UK regulators.
It is now being reviewed by the National Institute for Health and Care Excellence (Nice), which approves new NHS treatments in England, and final guidance on the drug is expected next year.
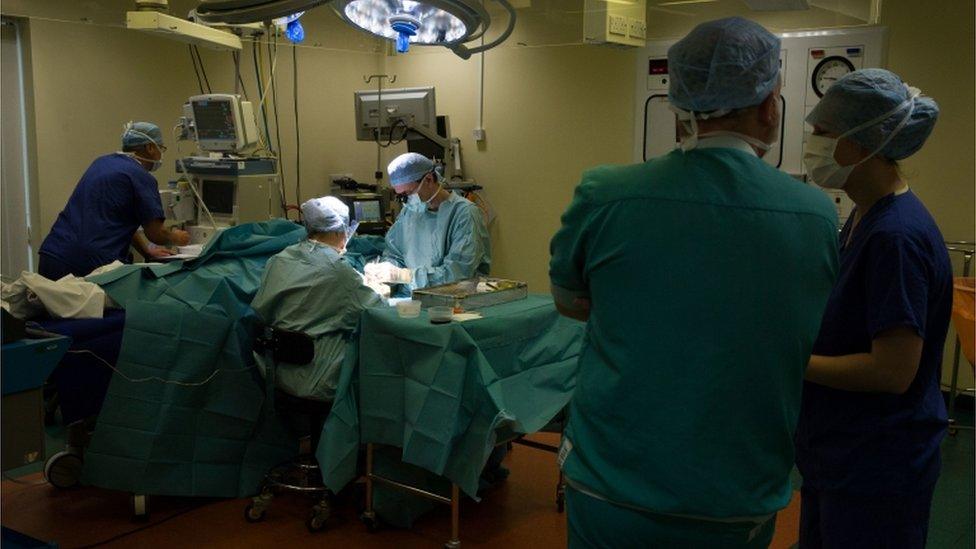
The delicate and highly-specialised nature of the hand surgery carried out by Carlos Heras-Palou meant hATTR amyloidosis would have stopped him from performing operations
About 100 people in the UK are believed to suffer from hATTR amyloidosis.
Mr Heras-Palou - whose sister Isabel also suffers from the disease and whose mother Catalina died from complications related to the conditions four years ago - underwent 18 months of treatment with patisiran, which has reversed the nerve damage that threatened to end his 20-year career.
Having been diagnosed early after suffering "pins and needles and intermittent numbness", he said the "ground-breaking" treatment has been "like the proverbial silver bullet".
"The treatment has saved my career, and my life," he said.
"I'm left with some numbness in my feet and weakness, and I still can't run, but I can walk. I'm functioning very well.
"I've been given hope and I'm seeing life in a different way."
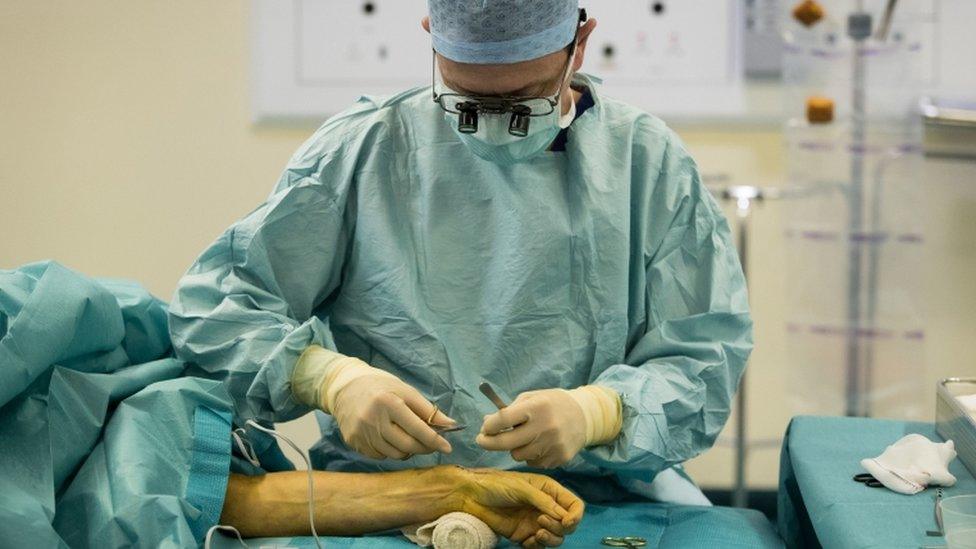
Carlos Heras-Palou said he is "functioning very well" after receiving patisiran as part of the treatment's trial
The trial involved 148 patients being treated with patisiran and 77 with a dummy placebo, and a report on the research published in the New England Journal of Medicine said disease progression was "halted or reversed" in patients who received the active drug.
As a trial participant, Mr Heras-Palou has been allowed to continue receiving patisiran, which is currently priced at about £308,618 ($400,000) per patient per year.
Professor Philip Hawkins, director of the National Amyloidosis Centre and one of the scientists involved in the trial, said the results were "wonderfully encouraging".

Follow BBC East Midlands on Facebook, external, Twitter, external, or Instagram, external. Send your story ideas to eastmidsnews@bbc.co.uk, external.