Covid trial volunteer left unable to prove double vaccination
- Published

Jo Wiggans received two doses of the Novavax vaccine at the end of 2020
A volunteer in a Covid-19 vaccine trial has said it has left her unable to travel and "invisible on the system".
Jo Wiggans, from Littleborough in Rochdale, received two doses of the Novavax vaccine at the end of 2020.
As the jab has not been licensed in the UK, the 70-year-old said it meant she could not show she had been vaccinated.
The UK's Deputy Chief Medical Officer Prof Jonathan Van-Tam has said, external the government is working to ensure trial participants were not "disadvantaged".
Mrs Wiggans volunteered to take part in the Novavak trials in November and December and said she had not been warned about any travel implications at the time.
In January, the US-based firm posted positive results for its UK trial and the government later confirmed 60 million doses were set to be produced at a factory in Stockton-on-Tees, but the vaccine is yet to be approved in the UK.
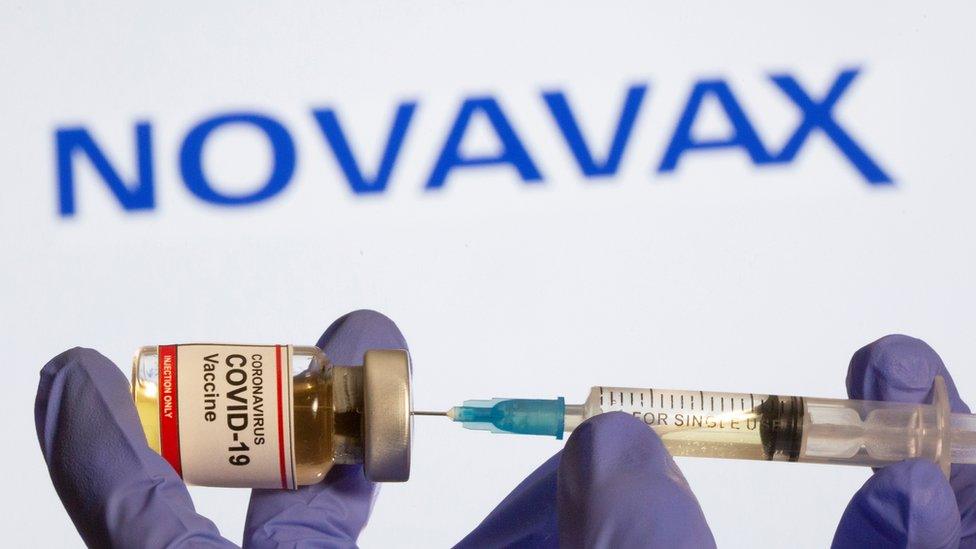
Novavax was shown to be 89.3% effective at preventing Covid in its Phase 3 UK trial
Mrs Wiggans told BBC North West Tonight she had been due to travel to see family in France, but could not enter the country.
"My husband can go, because he had the Pfizer vaccine," she said.
"I thought I was doing a good thing, but it's not on my record.
"I'm invisible on the system and the result is that France won't let me in, because I cannot provide evidence that I have been vaccinated."
Mrs Wiggans said she did not regret volunteering, but she "never, ever expected to be in a disadvantaged position".
"I really think this is unjust and massively unfair," she added.
The UK is currently on the French amber list, external, meaning who is not fully vaccinated is only permitted to enter the country for essential reasons.

Professor Jonathan Van-Tam said "individual countries control their own policies and exemptions"
In an open letter in June, Prof Van-Tam said the UK government's position was that "a vaccine should not be a requirement for travel".
He said "clearly, individual countries control their own policies and exemptions", but discussions were "ongoing with other countries... to shape the approach taken around the world to sharing health status for travel, including vaccination status", and the G7 nations, which includes France, had "committed to that approach".
In a statement issued in June,, external Novavax said it "firmly believes that clinical trial participants should not be disadvantaged with respect to providing proof of vaccination".
"We continue to work with the National Health Service, Vaccine Task Force and National Institute for Health Research, so that those who received [the vaccine] will soon have their vaccination dates entered into the NHS app," the firm said.

Why not follow BBC North West on Facebook, external, Twitter, external and Instagram, external? You can also send story ideas to northwest.newsonline@bbc.co.uk, external
- Published6 July 2021

- Published3 July 2021

- Published26 June 2021

- Published29 March 2021
- Published29 January 2021

- Published29 January 2021