Call for North West volunteers in monkeypox vaccine trial
- Published
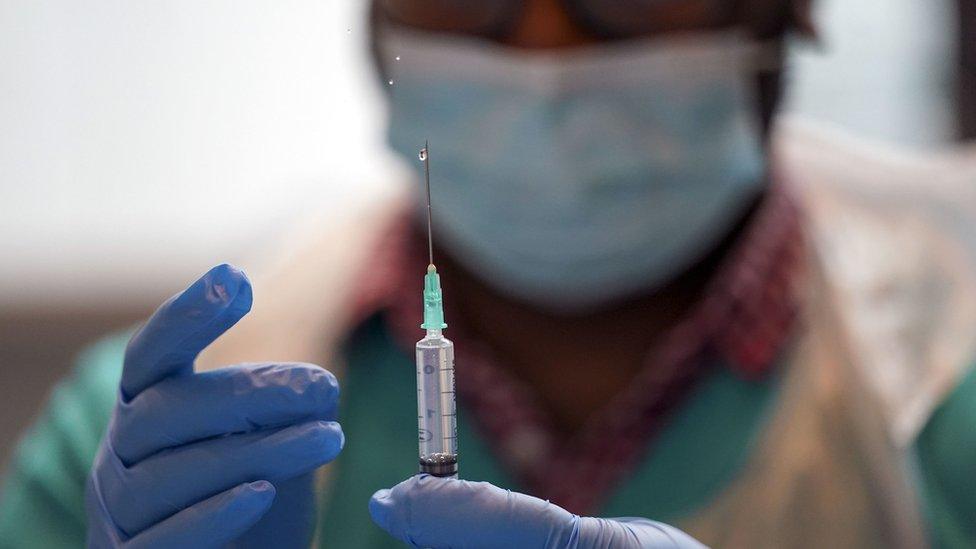
Volunteers from the north west of England are being asked to take place in a monkeypox vaccine trial
Volunteers from across the north west of England are being sought for trials of a new monkeypox vaccine.
The virus was endemic in parts of Africa until a global outbreak in 2022 saw cases spread to the UK.
The National Institute for Health and Care Research (NIHR) is calling for healthy people between 18 and 49 to come forward.
The study is funded by American biotechnology firm Moderna, which produced one of the Covid-19 vaccines.
The virus, now known as mpox by medical professionals, can cause a rash that develops into fluid filled blisters, which tend to eventually crust over and fall off.
Other symptoms include a fever, sore-throat, swollen lymph-nodes, back pain, headaches and low-energy.
Currently only one monkeypox vaccine is licensed, and the trial is seeking to establish how effective a new jab would be.
Vulnerable groups
Experts said most people infected with the disease could feel quite unwell for three to four weeks, but should make a full recovery.
However Professor Richard Fitzgerald, principal investigator for the trial based in NIHR's clinical research facility in the Royal Liverpool Hospital, said the virus could cause problems for vulnerable groups.
Speaking to BBC Radio Merseyside, he said: "If that was that, then that would be fine. It would be something we would have to get used and deal with as part of day-to-day life like we do with a lot of viruses.
"But the problem is there are groups of people, particularly people who are really young, people who are pregnant, and people who have lots of other medical problems where their immune system may not be so great at fighting off infection when mpox can be a lot more severe."
Prof Fitzgerald said in those vulnerable groups the virus had been known to cause serious conditions including damage to the eyes risking sight loss, and could cause dangerous swelling of the brain.
The World Health Organization declared monkeypox a global health emergency in 2022
He also told the BBC experts had noted a change in the way once solely tropical viruses such as dengue and zika spread globally, possibly fuelled by greater connectivity and travel.
The NIHR said volunteers would be screened and then receive two to three doses of either the investigatory vaccine or a placebo.
Mohsina Yousif, a teaching assistant in Liverpool, volunteered for the study after seeing a poster in a leisure centre.
The 44-year-old is also originally from Sudan, where the first cases were identified after the World Health Organisation (WHO) declared monkeypox as a global health emergency in July 2022.
She said: "I did it to help the NHS and to help people. I like to volunteer and help, and being part of this trial was a great way to do that."

Mohsina Yousif signed up after seeing a poster in a local leisure centre
Daniel Rawson, a milkman from Stockport, is also involved in the trial.
He said: "My message would be that people are needed to actually help with the trial, so if you're able to, please come along and give a helping hand."
The study is the first that Moderna is running entirely in the UK as part of a 10-year partnership with the government aimed at developing vaccine technology.
Dr Rajeka Lazarus, national co-ordinating investigator for the study, added: "Mpox is a global public health threat, and more vaccines are urgently needed to prevent future outbreaks.
"Throughout the Covid-19 pandemic, we were overwhelmed with the generosity of volunteers who came forward to take part in a number of vaccine trials. Without them, the advances we've seen would not have been possible. It would be fantastic to see the same support for mpox research."

Why not follow BBC Merseyside on Facebook, external, X, external and Instagram, external? You can also send story ideas to northwest.newsonline@bbc.co.uk, external
- Published5 August 2022