Coronavirus: NI 'could receive 570,000 doses of vaccine'
- Published
- comments
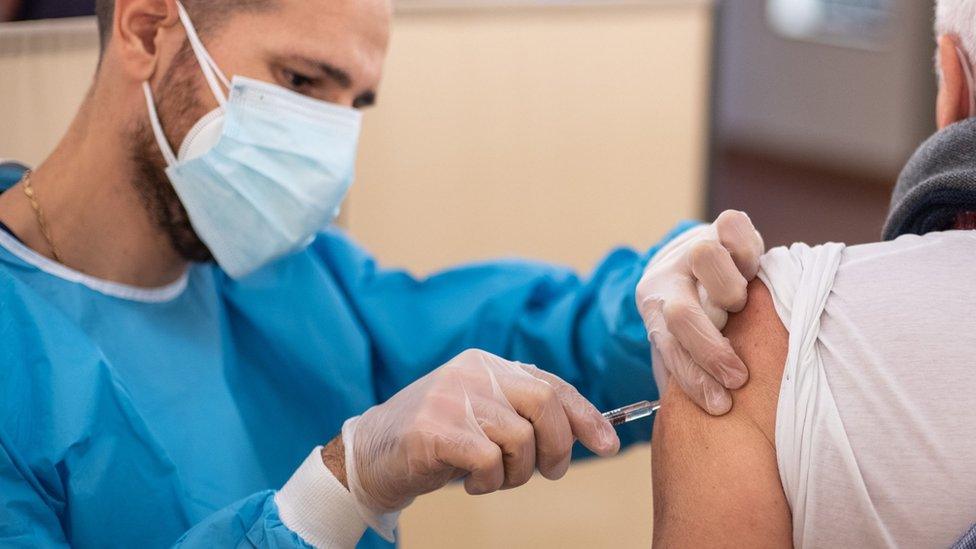
Vaccination for those at most risk from Covid-19 could begin by the end of the year, according to NI's health minister
NI is likely to receive about 570,000 doses of the new coronavirus vaccine if it passes the next stage of trials and becomes licensed.
This means that 285,000 people could potentially be vaccinated for Covid-19.
The Department of Health told BBC News NI that the local supply will be part of a UK order and distributed among the regions using the Barnett Formula.
It said that the first 20 million doses of the vaccine is scheduled to be in the UK by the end of next March.
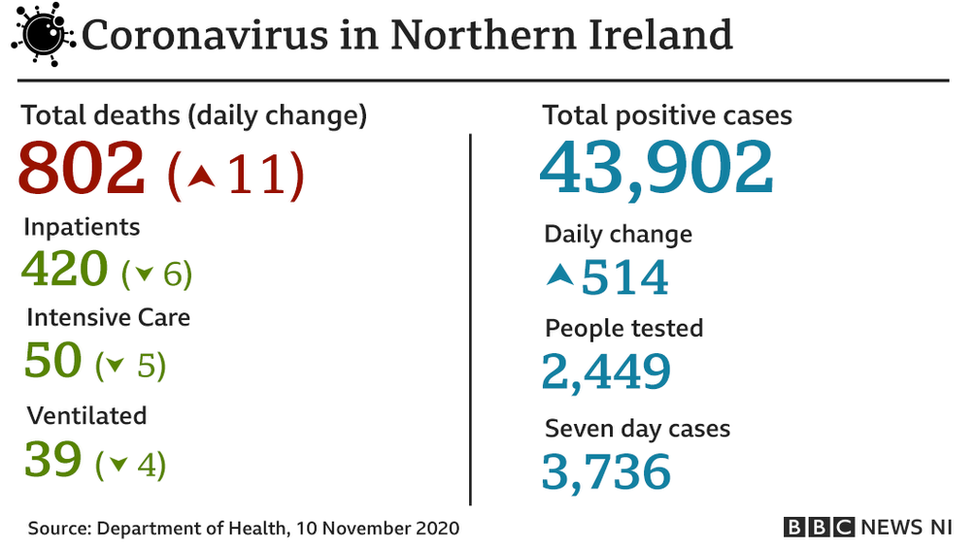
On Tuesday, the Department of Health, external announced a further 11 coronavirus-related deaths in Northern Ireland and 514 more positive cases.
Sixteen deaths related to Covid-19 were announced in the Republic of Ireland on Tuesday, 14 of which occurred in November, one in October and one that "remains under investigation". There were a further 270 confirmed cases of the virus.
Northern Ireland's Health Minister Robinson Swann said that while the vaccine was "good news", it still needed to officially pass phase three of its trials and be authorised by the regulator.
On Monday, he said vaccination for those most at risk from Covid-19 could begin by the end of the year.
Dr Tom Black, chair of the BMA in Northern Ireland, said the most vulnerable should get the vaccine first.
"We've 146 nursing home outbreaks in Northern Ireland today, so nursing homes have got to be a priority," he told BBC Radio Ulster's Evening Extra programme.
"Those over 85, those most vulnerable with underlying conditions and obviously the health care staff, because we've been losing a lot of health care staff to illness and isolation and we'd like to get them it because the intensive care units and the wards are very busy."
Dr Black said the logistics to get 570,000 doses of the vaccine rolled out would take "some amount of work" but could be done "so many at a time - so the first cohort will be the over-85s".
The vaccine - developed by Pfizer and BioNTech - was tested on 43,500 people in six countries and no safety concerns have been raised.

Robin Swann said there was "intense" pressure on the health service
The data shows that two doses, three weeks apart, are needed for the vaccine to work.
Meanwhile, the health minister has said the vast majority of track and trace cases are being successfully detected.
In the past week, a total of 4,450 cases have been sent to test, trace and protect and 4,023 cases were successfully detected, Robin Swann added.
He told the assembly there were a further 9,267 contacts from these cases and that 99% of those were contacted.
- Published9 November 2020

- Published9 November 2020
- Published28 May 2021
- Published9 November 2020