Medicine supply chains into NI post-Brexit a 'high risk area'
- Published
The chief pharmaceutical officer has warned that without mitigation, the supply of medicines and medical devices into Northern Ireland would be considered a "very high risk area".
Cathy Harrison was speaking to the NI health committee about the implications of Brexit and the NI Protocol for the pharmaceutical industry.
The industry currently has a 12-month grace period to make preparations.
Until this year, there has been no separate supply chain for NI.
It was treated as part of the UK supply chain.
Ms Harrison told the committee an "enormous amount of work" was being done in the area.
"Without us taking action, and without a very proactive approach being taken, and if we did nothing at all until the end of this year, then there would be a high level of risk and it would be considered a very high risk area," she said.
"However, there's an enormous amount of work going on to avoid that.
"Ahead of the overall transition of the UK from the EU, there was a massive amount of work done with the medical supply chains.
"That's really now standing us in good stead."
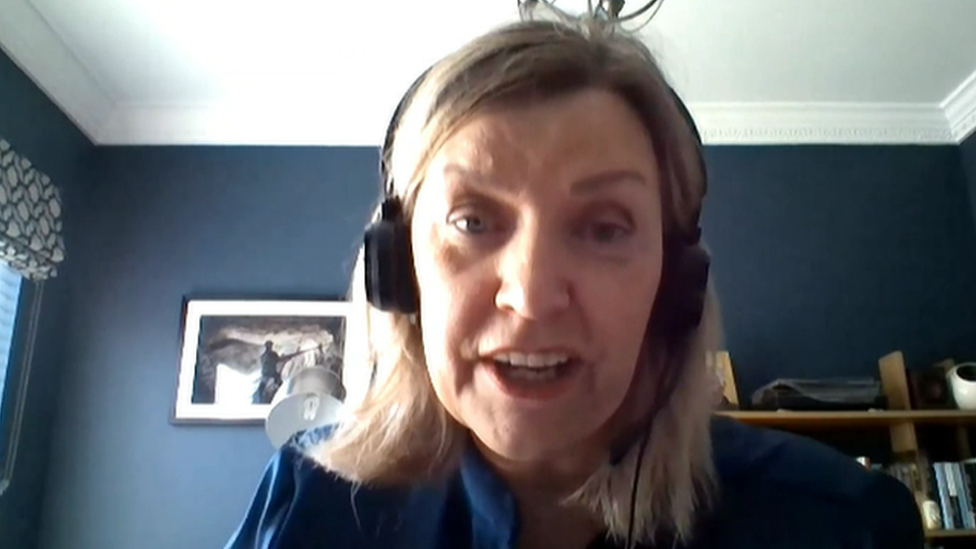
Cathy Harrison speaking to the NI Health Committee on Thursday
But she acknowledged that costs may rise.
"Essentially, Northern Ireland is now a very small market globally for pharmaceuticals and for medical devices," she said.
"I have to say, there is a very high level of commitment from the parties I've been involved with in the pharmaceutical industry, in terms of working towards a new normal, if you like, for Northern Ireland, but there's a recognition that in doing that, our costs are likely to increase."
Many medical devices, and about £600m of medicines, are brought into Northern Ireland every year, with around 98% coming from Great Britain.
'Disaster'
The committee also heard that the health minister is to be given options concerning the Cross-Border Healthcare Directive. , external
The directive stopped after the Brexit transition period ended on 31 December 2020.
It had allowed people in Northern Ireland to pay upfront for treatment in an European Economic Area (EEA) country, then claim reimbursement from the Health and Social Care Board (HSCB).
The committee chairman, Colm Gildernew, described it as a "disaster".
"Given the fact of the waiting list situation that we have currently, is this not a disaster? Should this not be a priority, to get something to replace that cross-border directive urgently in place?," he said.
Following the meeting, a Department of Health official said: "The options around the continuation or not of the Cross Border Healthcare Directive in the immediate term are to be provided to the minister imminently and those options will be considered before any announcements are made."
- Published11 November 2020