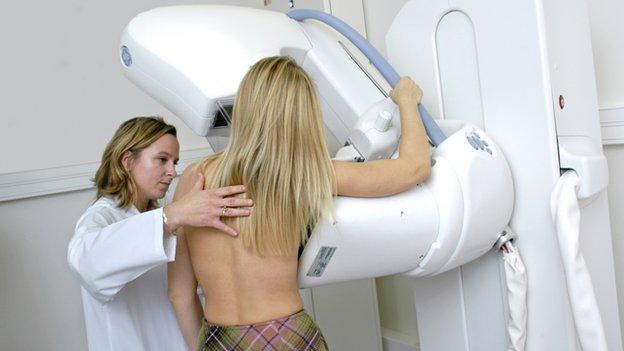
Breast cancer drug Perjeta refused for use in Scotland
- Published

Breast cancer drug Perjeta was approved for use in England and Wales in 2015
Breast cancer sufferers in Scotland have been denied routine access to a treatment which is available to patients south of the border.
The drug Perjeta can reduce the need for mastectomies among breast cancer patients.
But the Scottish Medicines Consortium (SMC) recommended it was not routinely used by the NHS amid concerns about long-term survival benefits.
Campaigners branded the move "the ultimate postcode lottery".
The drug, which is also known as pertuzumab, can treat an aggressive type of breast cancer known as HER2-positive.
Around 15% of people diagnosed with early-stage breast cancer have HER2-positive tumours.
Perjeta was approved for use in England and Wales in September 2015, where it is used to shrink and control tumours before surgery.
After announcing its decision, the SMC said it would meet the pharmaceutical company behind the drug, Roche, to discuss "next steps", external.
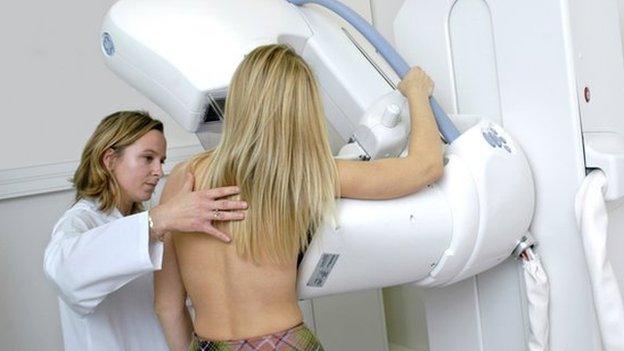
Perjeta is used to shrink tumours ahead of surgery and it can reduce the need for a mastectomy
Nicolas White, of Breast Cancer Care, told BBC Radio Scotland that the drug could have benefited around 400 people in Scotland.
Speaking on the Good Morning Scotland programme, he said the regulator's decision was "disappointing and confusing".
"If you're diagnosed with this particular type of breast cancer and you live in Gretna, you wouldn't benefit from this treatment," he added.
"Yet only a few miles down the road in Carlisle, if you were given the same diagnosis, you would benefit from this treatment.
"It's the ultimate postcode lottery, really, a cross-border postcode lottery."
It follows a similar controversial decision in September 2014, when the SMC decided not to approve Kadcyla, another breast cancer drug.
Difficult decisions
More than 3,400 women in England have used Perjeta and Kadclya via the Cancer Drugs Fund, according to Roche.
The drug company's general manager Richard Ewin said: "Today's decision is a devastating blow to women in Scotland with this very aggressive form of breast cancer.
"This means that these women will be denied this early-stage treatment which allows for reduction of large tumours to a size that is operable, potentially enabling breast conservation surgery instead of mastectomies."
Perjeta was one of four drugs the SMC rejected for routine use on the NHS, although it did back a new treatment Jevtana - also known as cabazitaxel - for patients with advance prostate cancer.
SMC chairman Professor Jonathan Fox said he knew some decisions would be "hard for patient groups and clinicians".
"However, when we considered all the evidence in front of us, it was not strong enough for us to be able to accept these medicines for routine use," he added.
The SMC approved the routine use of a drug used to treat prostate cancer
Jevtana was initially rejected by the SMC in 2011 and again earlier this year.
The move was welcomed by Prostate Cancer UK, which has campaigned to give men in Scotland access to the drug.
Heather Blake, the charity's director of support and influencing, said: "Today's approval of cabazitaxel chemotherapy is welcome news, and represents an important milestone for men with prostate cancer in Scotland.
"This is the last in a string of drugs which we have had to fight to be made available throughout the UK to those who would benefit.
"It means men in Scotland will now be routinely offered the same suite of treatments as those in other parts of the UK, ending variation in access which has been an ongoing injustice for years."
- Published13 June 2016

- Published29 September 2015
- Published25 September 2015
- Published13 October 2014