Coronavirus: Germany rejects 'mixed up' reports on AstraZeneca jab
- Published

Germany has begun its mass vaccination campaign as the virus spreads
Germany's health ministry and AstraZeneca have rejected German media reports on Monday that cast doubt on the effectiveness of the drug-maker's vaccine in the elderly.
AstraZeneca, a UK-Swedish company, described the reports as "completely incorrect" while the health ministry said key data had been misreported.
The media reports quoted anonymous German government sources.
They come amid tension between the EU and AstraZeneca over vaccine supplies.
The European Union has said it may restrict exports of vaccines made in the bloc in response to AstraZeneca saying it cannot supply the agreed numbers of doses due to production problems.
What did the reports say?
The reports emerged in Handelsblatt, a respected financial daily, and the mass-circulation Bild, quoting sources within Germany's governing coalition.
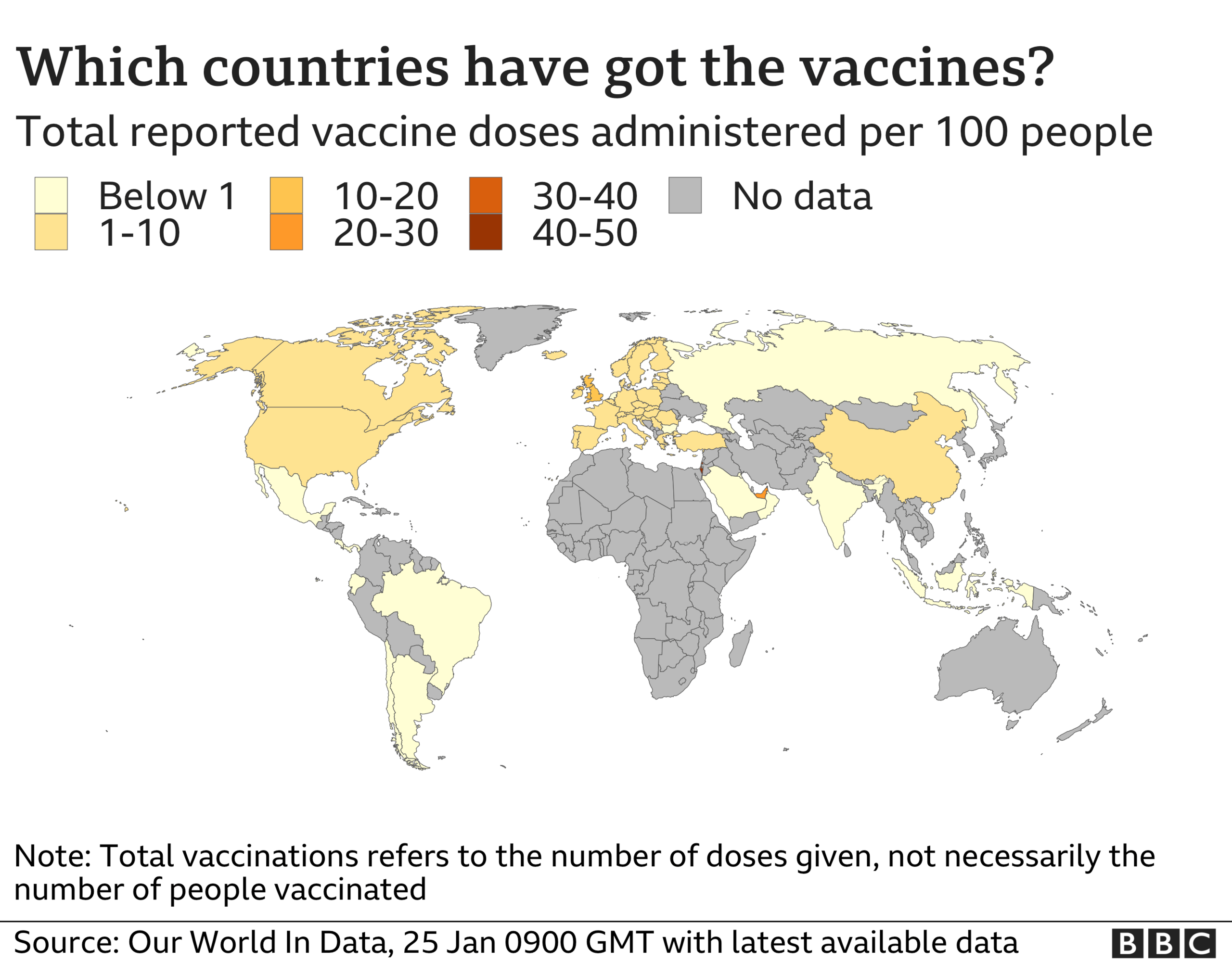
Germany and the wider EU are relying on millions of doses of the AstraZeneca product, alongside the BioNTech and Moderna vaccines, to fight the coronavirus pandemic.
Handelsblatt reported that the German government was working on the basis of only 8% efficacy among those over 65, compared with over 90% for the other vaccines.

Vaccination centres have been established throughout Germany
The AstraZeneca jab is expected to be approved by the EU's medicine's regulator, the EMA, in the next few days. But Bild suggested that it may not be approved by the EMA for use in the elderly.
The EMA's head, Emer Cooke, has been asked by MEPs about the efficacy of the vaccine in older people. She said studies carried out so far were on a very small quantity of the elderly population.
Her agency, she said, what trying to work out "what the data means for the populations that were studied and what can be expected for populations that have not been studied".
EMA approval, she added, could be granted for "a particular age group or for a wider age group".
How did AstraZeneca and the German government respond?
AstraZeneca said: "Reports that the AstraZeneca-Oxford vaccine efficacy is as low as 8% in adults over 65 years are completely incorrect."
In November, the firm had published data in the leading medical journal The Lancet "demonstrating that older adults showed strong immune responses to the vaccine, with 100% of older adults generating spike-specific antibodies after the second dose".
AstraZeneca chief Pascal Soriot, in an interview with Italy's La Repubblica, explained that the Oxford team started vaccinating older people later, external so it did not have "a huge number of older people that had been vaccinated".
"But we have strong data showing very strong antibody production against the virus in the elderly, similar to what we see in younger people."
He said it was possible that some countries "out of caution" would use the AstraZeneca vaccine just for younger people.
Full trial data in December showed the vaccine was 70% effective in all adults, and it was approved for use by the UK's MHRA regulator.
Reacting to Monday's German media coverage, the German health ministry said on Tuesday that it "appears two things have been mixed up in the reports" and that 8% actually referred to the number of volunteers of a certain age taking part in the study - not the efficacy rate.
"Around 8% of the volunteers in AstraZeneca's efficacy studies were between 56 and 69 years old and 3-4% are above 70 years old," said the ministry in a statement.
"However, this does not mean that it is effective only in 8% of older people."
The statement also noted that "fewer older people were involved in AstraZeneca's first studies than in those of other manufacturers".
Health Minister Jens Spahn, interviewed by German radio, refused to be drawn on what he called "speculation" and said he would await the EU regulator's decision.
- Published23 January 2021
- Published22 January 2021