Pfizer acts to stop its drugs being used in lethal injections
- Published

America's prisons have found it increasingly difficult in recent years to obtain the drugs needed to carry out executions
It has emerged that the largest US pharmaceutical company, Pfizer, recently took steps to prevent its drugs being used in lethal injections.
"We strongly object to the use of any of our products in the lethal injection process for capital punishment," the company said, external.
It stressed that its products were meant to save the lives of patients.
The move reportedly shuts off the last remaining open market source of drugs used in executions in the US.

America's most common form of execution: David Willis, BBC News, Los Angeles
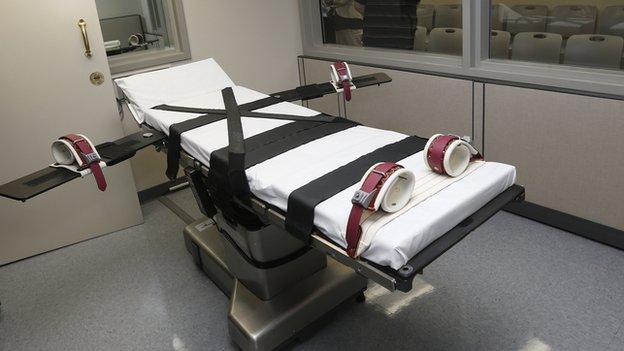
Lethal injection has long been the most common form of execution in the US.
Traditionally states relied upon a combination of drugs to put the inmate to sleep and restrict his breathing, leading to a cardiac arrest.
A European Union ban on the export of such drugs in 2011 prompted American drug manufacturers to follow suit, leaving officials in search of new drug cocktails by which the lethal injection could be achieved.
Two years ago an Oklahoma inmate - Clayton Lockett - took 43 minutes to die after being administered an untested mixture of drugs.

The decision follows similar moves by more than 20 US and global drug-makers, according to a New York Times report, external.
In a statement, external published on its website last month, Pfizer said seven of its drugs would only be sold to purchasers on condition that they would not resell them to correctional institutions.
Pfizer said it offered the products because they saved or improved lives, and marketed them solely for use as indicated in the product labelling.
"Pfizer makes its products solely to enhance and save the lives of the patients we serve," it said.
"We are committed to ensuring that our products remain available and accessible to the medical professionals and patients who rely upon them every day."
Human rights groups have long campaigned against using medicines for the purpose of capital punishment.
- Published8 June 2015
- Published3 February 2016

- Published1 October 2015
