Covid: Moderna vaccine moves closer to US approval
- Published

Six million doses could be ready to ship as soon as the vaccine gets FDA approval
A second coronavirus vaccine is nearing emergency approval in the US after it was endorsed by a panel of experts.
The head of the Food and Drug Administration (FDA) said his agency would move quickly to authorise the Moderna vaccine, allowing the company to begin shipping millions of doses.
President Donald Trump tweeted incorrectly that the vaccine had already been "overwhelmingly approved".
The Pfizer/BioNTech vaccine was approved earlier.
America has recorded more Covid-19 cases and deaths than any other and earlier this week, its death toll passed 300,000.
The advisory panel on Thursday voted 20-0 with one abstention that the benefits of the Moderna vaccine outweighed the risks for those aged 18 and over. The same committee last week backed the Pfizer/BioNTech vaccine, leading to its authorisation for emergency use the following day.
US Covid vaccine: Three key questions answered
Following the panel's endorsement, FDA commissioner Stephen Hahn said his agency had informed Moderna it would work "rapidly" towards issuing emergency use authorisation.
Yet Mr Trump posted without confirmation from the FDA that the vaccine had been "overwhelmingly approved" and distribution would "start immediately".
Allow X content?
This article contains content provided by X. We ask for your permission before anything is loaded, as they may be using cookies and other technologies. You may want to read X’s cookie policy, external and privacy policy, external before accepting. To view this content choose ‘accept and continue’.

Regulators reported earlier this week that the Moderna vaccine was safe and 94% effective.
The US has agreed to purchase 200 million doses, and six million could be ready to ship as soon as the vaccine gets FDA approval.
"To go from having a sequence of a virus in January to having two vaccines available in December is a remarkable achievement," said Dr James Hildreth, a member of the expert panel and CEO of Meharry Medical College in Tennessee.
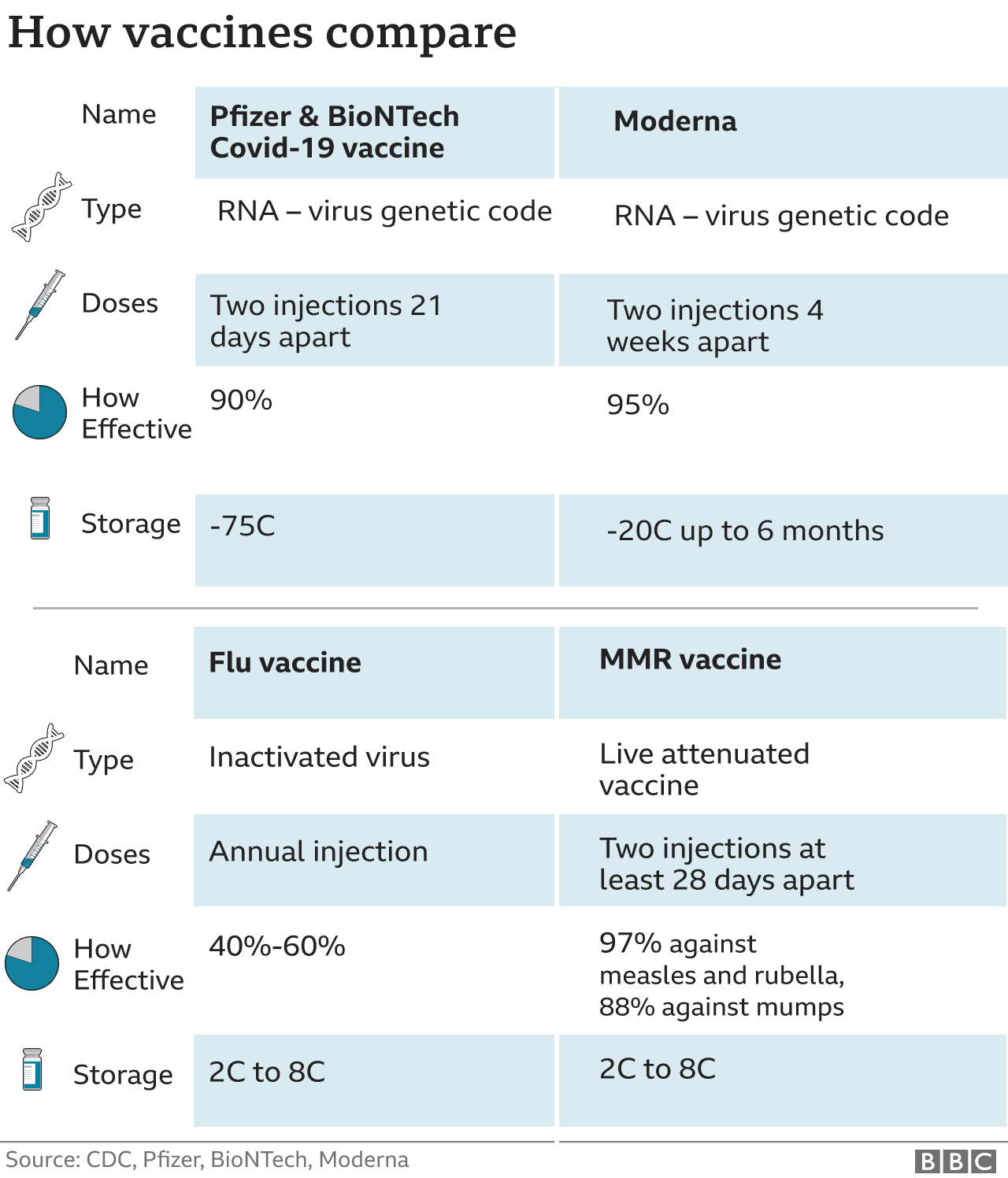
How does Moderna differ from the Pfizer jab?
It requires temperatures of around -20C for shipping - similar to a normal freezer.
The Pfizer jab requires temperatures closer to -75C, making transport logistics much more difficult.
Like the Pfizer jab, the Moderna vaccine also requires a second booster shot. Moderna's second injection comes 28 days after the first.

The company is based in Cambridge, Massachusetts, and has said that if approved, the "vast majority" of its doses would be manufactured there.
Pfizer's drug is being manufactured in several countries, including Germany and Belgium.
Other countries have also ordered the Moderna vaccine:
In Canada, the government plans to get two million doses by March - part of a total 56 million doses
The UK has already pre-ordered seven million doses
The European Union last month announced a contract to purchase of 80 million doses - with an option to purchase up to 80 million more - once the vaccine is deemed safe and effective
Japan has signed up for 50 million doses, South Korea for 20 million, and Switzerland has ordered 7.5 million, according to data compiled by the Duke University Global Health Innovation Center
Who is first in line in the US?
The US began its Covid-19 vaccination drive earlier this week, after emergency approval was given to the vaccine developed by US pharmaceutical giant Pfizer and its German partner BioNTech.
The vaccination programme aims to reach 100 million people by April.
New York nurse Sandra Lindsay was among the first people in the country to receive a coronavirus vaccine when the rollout began on Monday. Footage of her being vaccinated was streamed on the Twitter feed of New York Governor Andrew Cuomo, whose state was the epicentre of the US epidemic in the first wave earlier this year.
"I hope this marks the beginning of the end of a very painful time in our history. I want to instil public confidence that the vaccine is safe. We're in a pandemic and so we all need to do our part," Ms Lindsay said.
The day the US began Covid vaccinations
Centers for Disease Control and Prevention (CDC) guidelines submitted to US states say healthcare workers should be prioritised first, as well Americans living in long-term care homes.
Essential workers are expected to be next in line for the jab, but it will be up to states to decide which industries to prioritise.
Moncef Slaoui, the chief scientist of federal vaccine distribution programme Operation Warp Speed, says the young and healthy should be at the back of the queue.
At least 70% or 80% of the US population need to be vaccinated to achieve herd immunity, he said.
Related topics
- Published9 December 2020
- Published28 May 2021
- Published15 December 2020