AstraZeneca boss: I don't think I would do anything differently
- Published

AstraZeneca's chief executive Pascal Soriot
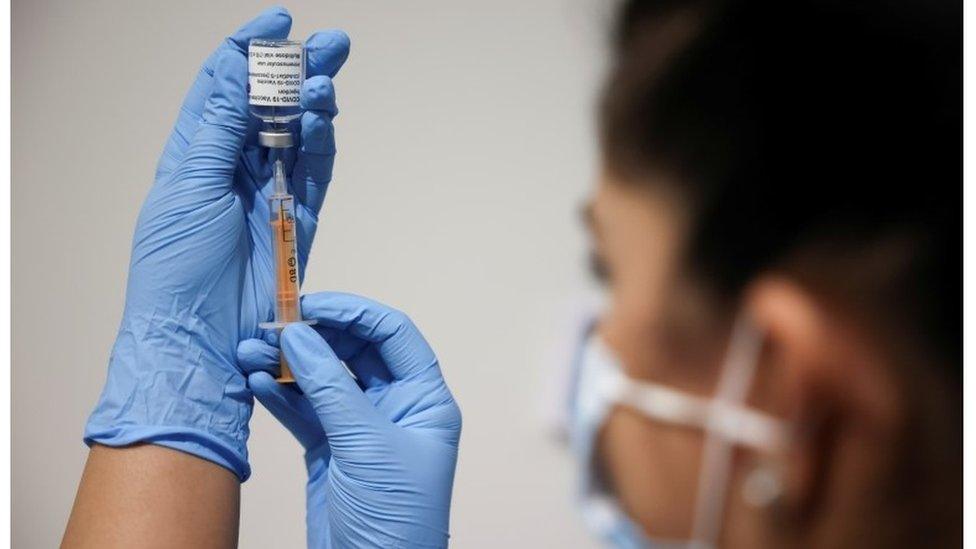
The head of the drugs giant behind the Oxford-AstraZeneca Covid-19 vaccine says the jab managed to save a million lives despite facing "setbacks".
AstraZeneca boss Pascal Soriot also addressed studies linking the vaccine to rare but dangerous blood clots.
Looking back on its development, he said: "I don't think I would do anything differently from what we did."
Many countries in Europe and Asia have placed age restrictions on the vaccine and the US has yet to approve it.
Mr Soriot received a knighthood in the Queen's Jubilee birthday honours last week for his contributions to science.
He was honoured for services to the UK in "life sciences and leadership in the global response to the Covid pandemic", AstraZeneca said in a statement on Wednesday.
Mr Soriot, who is chief executive of the British-Swedish firm, told the BBC during a recent visit to Singapore that the vaccine's quick development and distribution prevented a million people from dying of Covid-19.
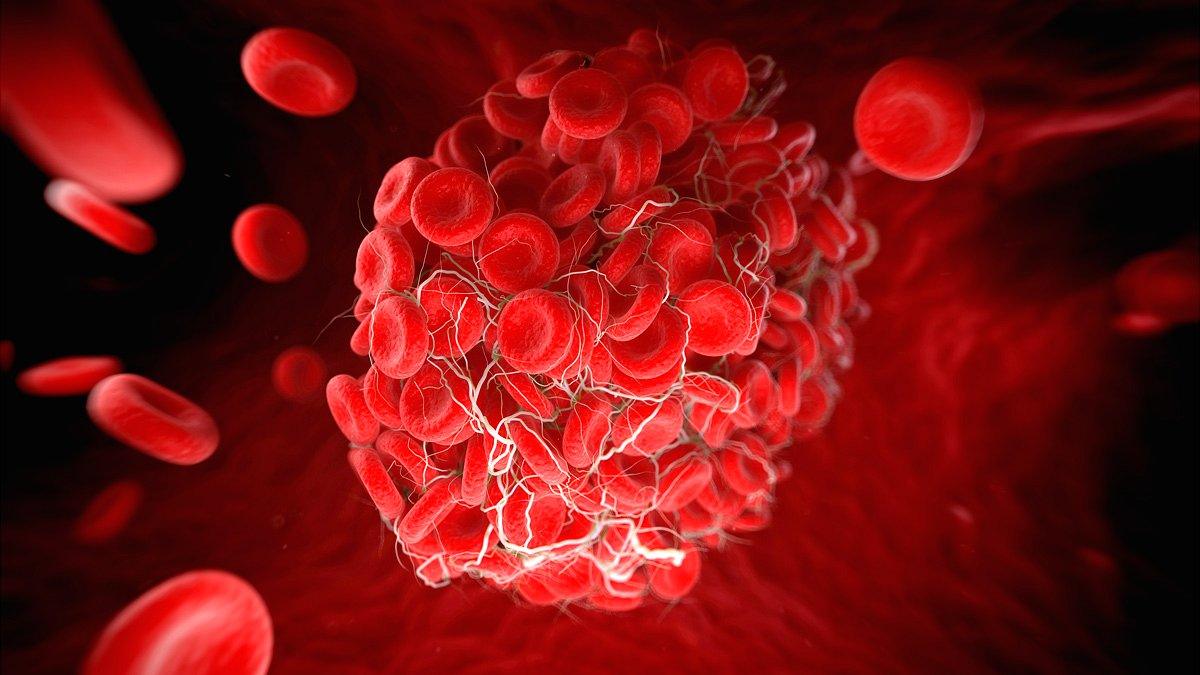
He said this came despite "setbacks" including concerns around rare but dangerous blood clots, which emerged last year.
"We decided to do it at no profit, we decided to partner with a network of partners around the world to scale up manufacturing. Despite the setbacks, we delivered three billion doses [of the vaccine] and saved a million lives," he said.
"When you launch yourself in something like this, which is a huge undertaking, you have to accept that you will have setbacks," he added.
AstraZeneca developed the vaccine in collaboration with the University of Oxford. It was first approved by the UK in December 2020 as countries raced to contain the growing numbers of coronavirus infections.
Nearly half of the adult population in the UK has received two doses of the vaccine, where it is believed to have saved more lives to date than the Pfizer and Moderna jabs combined.
Last year, UK regulators recommended the AstraZeneca jab for over-40s after its use was linked to extremely rare blood clots.
According to the UK's Medicines and Healthcare products Regulatory Agency, the risk of developing a blood clot was about four in one million.
However, many other European countries suspended their use of the vaccine. They only lifted their curbs and put age restrictions on the jab when European Union (EU) regulators declared that the benefits outweighed the risks.
The restrictions mean AstraZeneca's vaccine is now approved for use for a smaller segment of the population than several other Covid vaccines.
Mr Soriot said: "It is important to remember that those side effects are extremely rare. When you start vaccinating millions of people, very rare side effects will emerge that remain very rare. And this is common to all vaccines."
EU regulators only approved the vaccine's use as a "third dose booster" for adults last month.
Although the vaccine can be safely refrigerated for up to six months, several African states have destroyed or returned their stocks, as they said they could not use the jabs before they expired.
Meanwhile, Mr Soriot said in less developed economies - including in Asia - some people were reluctant to get vaccinated.
"In the emerging, developing countries, there's quite a bit of hesitancy. Of course, China is a different story where they're still managing a 'zero-Covid' policy. So it depends where you are in the world," he added.
Why do some vaccines protect you longer than others?
Mr Soriot said the firm was still in discussions with US authorities about submitting the vaccine for approval in the country, as the "need for a new vaccine is much less in the US than it was".
"Today, there is [an] over supply. We do have too many vaccines. So the question is how do we deliver? How do we administer those vaccines and how do we manage vaccine hesitancy? So we are in a very different place," Mr Soriot said.
In November, AstraZeneca said it will move away from providing its Covid vaccine to countries on a not-for-profit basis, as the disease was becoming endemic.
It said it expected to make a modest income from the vaccine from a series of for-profit agreements.
The jab will continue to be supplied on a not-for-profit basis to poorer countries.
- Published1 June 2022

- Published7 February 2022
- Published8 December 2021
- Published2 December 2021
- Published27 August 2021
- Published23 November 2020
