Glenfield couple's delight at 'most expensive' drug approval
- Published
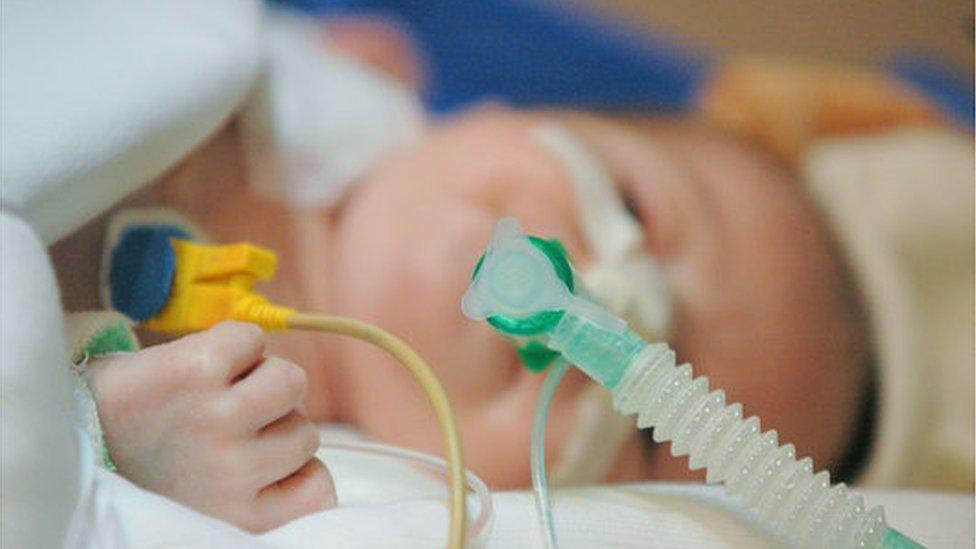
Metehan's parents were told babies with his condition do not survive past the age of two
A couple who raised £1.9m for a drug for their son have called its approval on the NHS "brilliant" - although it has come too late to treat him.
Metehan, from Glenfield, Leicestershire, has severe spinal muscular atrophy (SMA).
On Monday, the National Institute for Health and Care Excellence (Nice) sanctioned the use of Zolgensma - but only for babies up to 12 months.
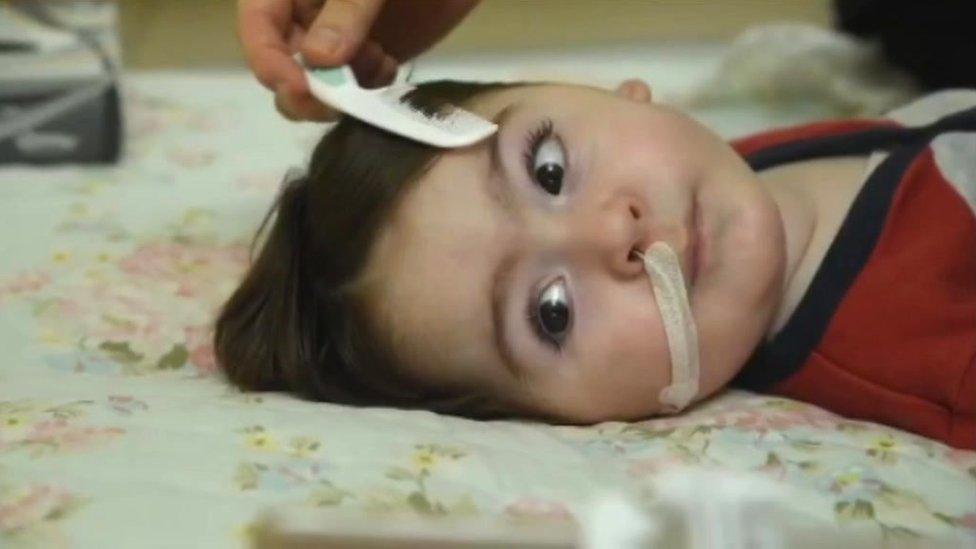
Metehan is nearly 16 months old and unlikely to be eligible.
However his parents Tuncay and Zeliha Fidan have raised £1.9m in donations to pay for him to receive Zolgensma at a hospital in Boston, Massachusetts.
The pair are still planning to take him to the US, despite the UK approval.
"A baby like Metehan is not easy to travel with - you have to consider everything - but at the same time it is really important he receives the treatment as soon as possible," Mr Fidan said.
"We have a plan in Boston and it's very clear.
"We're going to go there, get all the tests done and he's going to have the drug straight away."
He said the couple considered pushing the UK authorities to make an exception for their son but said: "We might spend two months chasing and we might end up not having it."
Metehan is nearly 16 months old so would not be eligible for Zolgensma in the UK
Metehan was diagnosed with SMA when he was seven weeks old.
His parents were told babies with his condition do not live beyond the age of two.
Zolgensma works by replacing the gene that causes the rare disorder.
With a list price of £1.79m, the one-off drug could become the most expensive ever approved by Nice.
'Raise everyone's voice'
Despite his disappointment, Mr Fidan said it is "brilliant" Zolgensma has been approved by the NHS.
He said part of the family's campaign was about raising awareness of the condition and hopes that influenced the decision to approve Zolgensma.
"It was on mainstream media so we did try to raise everyone's voice," he said.
A Nice spokesman said the evidence for the drug was limited to treatment at or younger than six months.

Follow BBC East Midlands on Facebook, external, Twitter, external, or Instagram, external. Send your story ideas to eastmidsnews@bbc.co.uk, external.
Related topics
- Published11 March 2021

- Published8 March 2021